
Understanding

Power
TDK-Lambda has a high level of expertise in the design, production and application of power supply solutions.
In particular, the terms MOOPs and MOPPs are often used in the context of AC-DC power
supplies for medical technology. The following article explains the meaning of these
terms and what needs to be considered in this area.
The introduction of EN 60601-1:2006 (edition 3), replacing the previous
EN 60601-1 :1990 standard, was a major change for the medical electrical
equipment and medical electrical systems industry. The transition to a hazard,
risk-based format meant that a power supply certified for medical applications
would no longer ensure that the end product would conform to the medical standard. This
is because medical equipment is now split into two categories; in vitro diagnostic medical
devices (IVD) and medical devices.
In vitro is Latin for “within the glass”, and these types of medical devices cover biological sample testing, to determine a person’s health for example. Most of these devices would not come into contact with a patient’s body and include blood or fluid analysers.
Medical devices though, may have direct connection to the patient, which raises the risk that the device could present a hazard. Examples of these types of electrical equipment are patient monitors, surgical tables, scanners and blanket warmers. They also include dental and healthcare equipment.
Manufacturers of medical devices will conduct risk management in accordance with ISO 14971 and record that information in their files.
The safety standard IEC/EN 60601-1 defines the minimum creepage and or clearance of the insulation depending upon the voltage.
The creepage distance is measured across the surface of an insulator and the clearance measured through the air between the primary and secondary sides of a power supply for example (Figure 1).
In vitro is Latin for “within the glass”, and these types of medical devices cover biological sample testing, to determine a person’s health for example. Most of these devices would not come into contact with a patient’s body and include blood or fluid analysers.
Medical devices though, may have direct connection to the patient, which raises the risk that the device could present a hazard. Examples of these types of electrical equipment are patient monitors, surgical tables, scanners and blanket warmers. They also include dental and healthcare equipment.
Manufacturers of medical devices will conduct risk management in accordance with ISO 14971 and record that information in their files.
Means of Protection (MOP)
A MOP prevents not only the patient receiving a hazardous electrical shock from a medical device, but also the operator. Two examples of a means of protection would be suitable insulation or a protective earth (PE) ground connection.The safety standard IEC/EN 60601-1 defines the minimum creepage and or clearance of the insulation depending upon the voltage.
The creepage distance is measured across the surface of an insulator and the clearance measured through the air between the primary and secondary sides of a power supply for example (Figure 1).
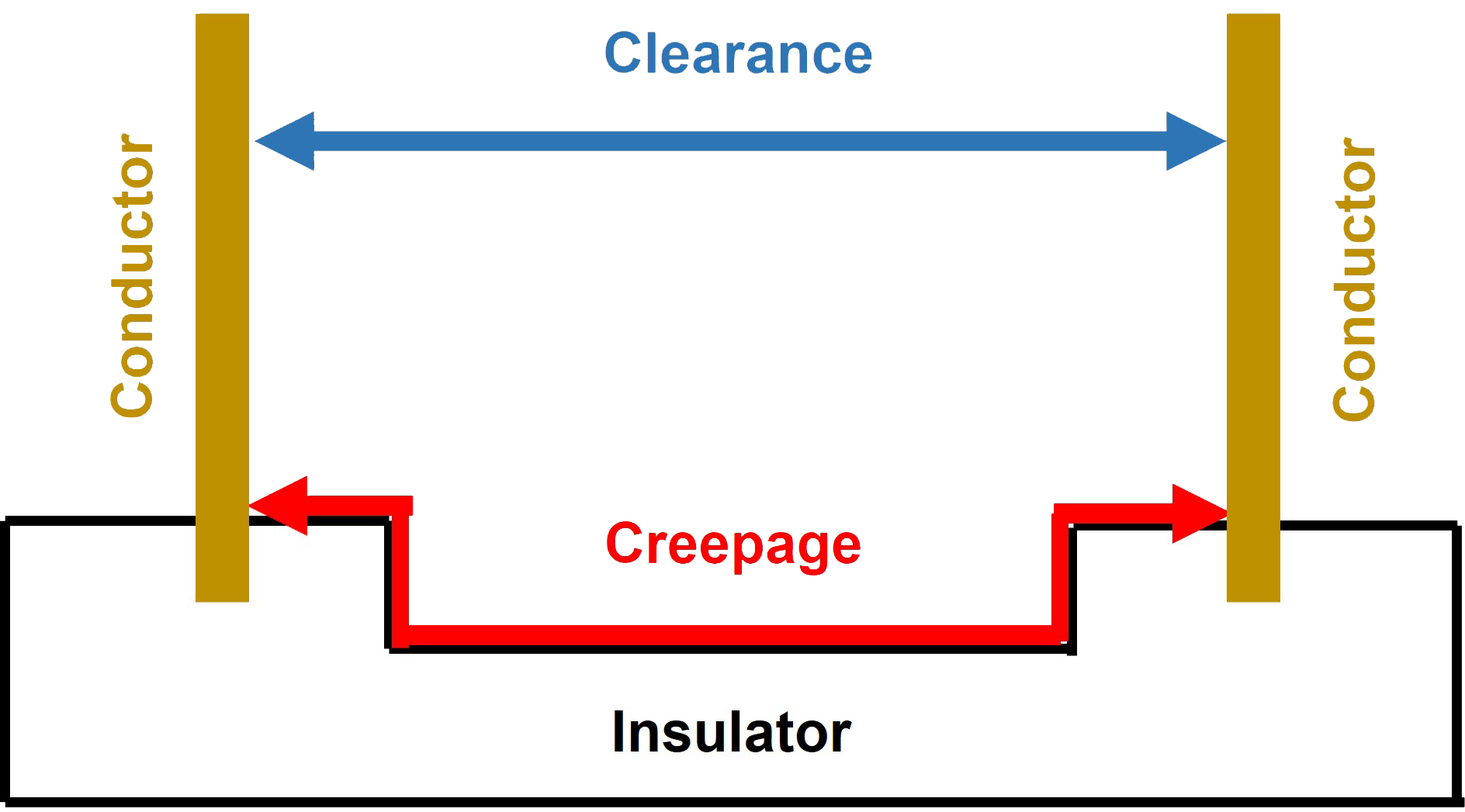
Figure 1: Creepage and clearance
×
![]()