In a recent announcement, the FDA released an article, “Medical Device Safety Action Plan: Protecting Patients,
Promoting Public Health,” that outlines various FDA actions to improve medical device safety. One of the
components of this plan is the call for mandatory built-in capabilities providing safety and security updates to
medical devices.
The Challenge
The challenge for medical device manufacturers is to securely, reliably, and cost-effectively build in these
safety and security update capabilities. Device manufacturers can no longer consider the development phase the
only or even the most significant cost driver in the product lifecycle. The ability to update is necessary to
meet new and evolving FDA and other regulatory requirements for medical devices. This use case outlines
solutions for designing such update capabilities into a medical device.
The Solution
With every problem comes opportunity. More sophisticated device manufacturers can see the new guidelines as an
opportunity to better understand their customers’ needs and better enable interaction with their customers over
the life of their product. As the use of medical devices by hospitals and patients continues to grow, new safety
concerns emerge that need to be dealt with to maintain patient health. Additionally, as new cybersecurity
vulnerabilities are discovered, they need to be mitigated to ensure continuing patient safety. Wind River ®
offers a portfolio of products, including the use of virtualization technology available with VxWorks®, Wind
River Linux, and Wind River Titanium Cloud™, that can help medical device
companies create reliable software
update capabilities in a cost-effective manner.
VxWorks
VxWorks is found in more than 2 billion devices in medical, industrial, transportation, and defense solutions.
Its small footprint enables devices to meet hard real-time operating requirements in scaling from small to large
medical devices. It works on all major processor architectures, Arm®, x86, and PowerPC.
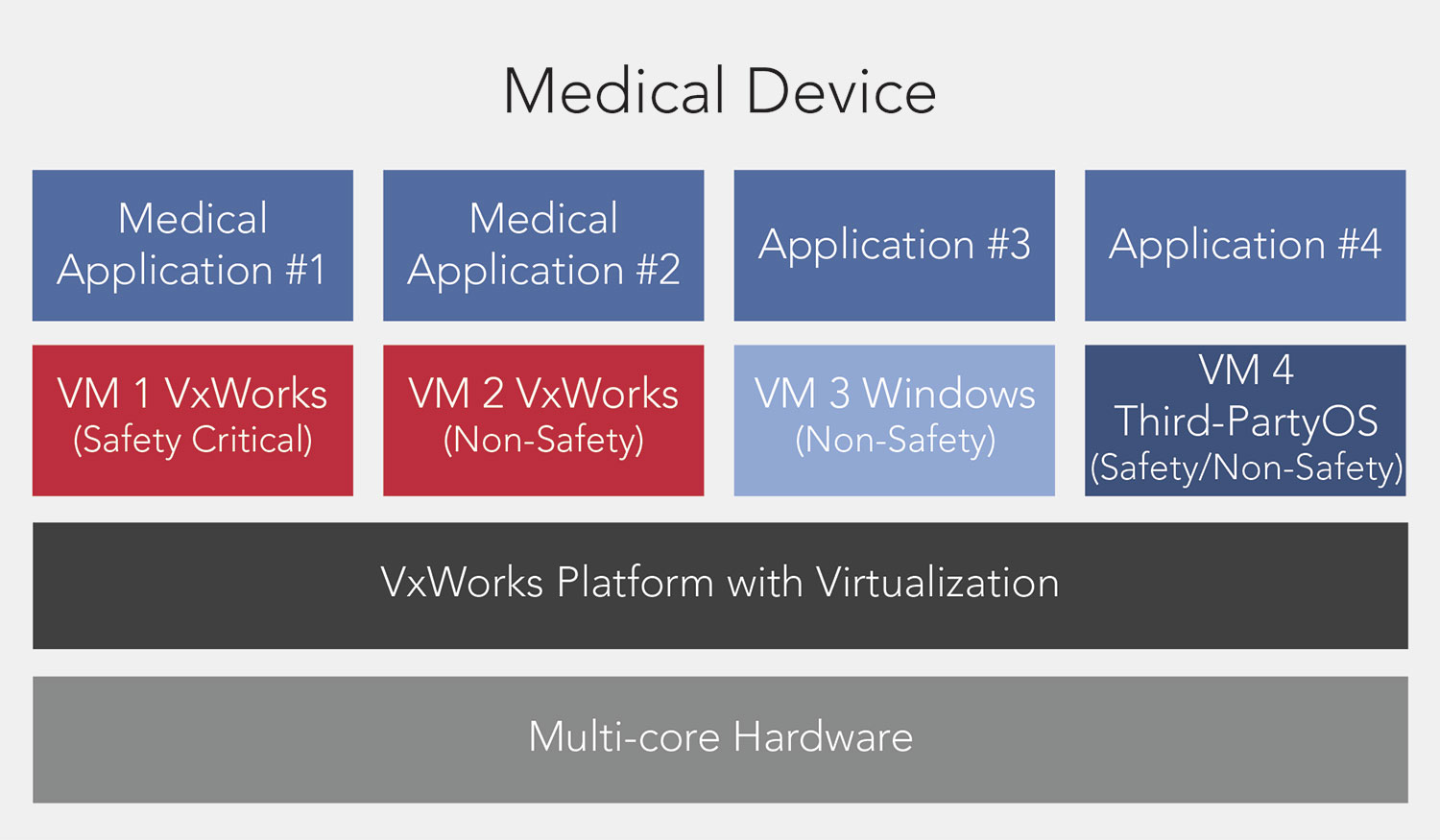
Example of a VxWorks platform with
virtualization running
medical applications in virtual machine partitions


